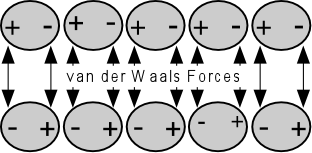
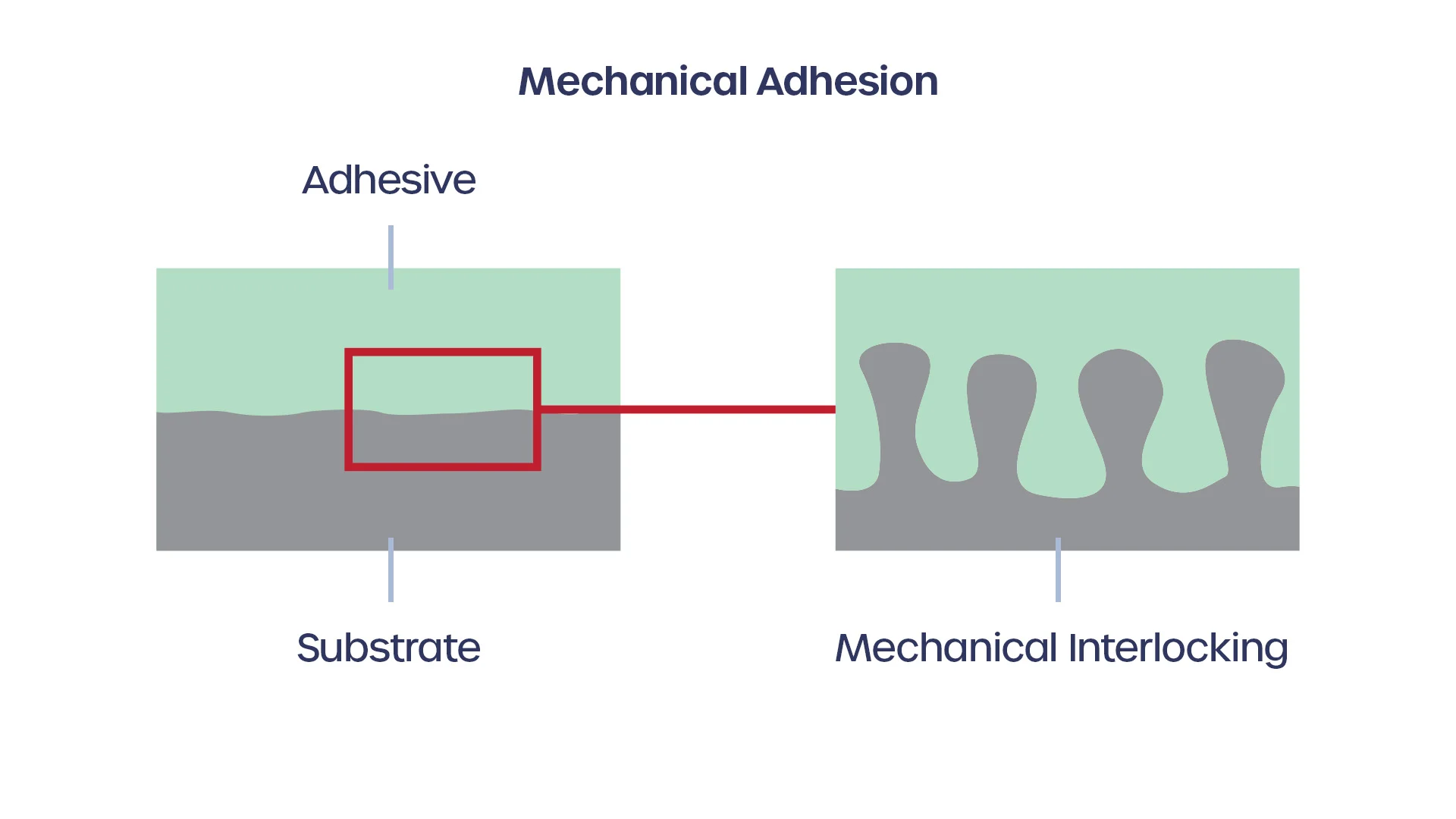
How easily the adhesive will “wet out” on the surface is a function of the relative adhesive and cohesive forces intrinsic to the type of contaminant and the type of surface. Cohesive forces are defined as the attraction between molecules of the same type while adhesive forces are the attraction between different molecule types. Cohesive forces will try to collapse the glue or contaminant into the smallest possible surface area while adhesive forces will cause it to flatten out on the surface of the substrate.
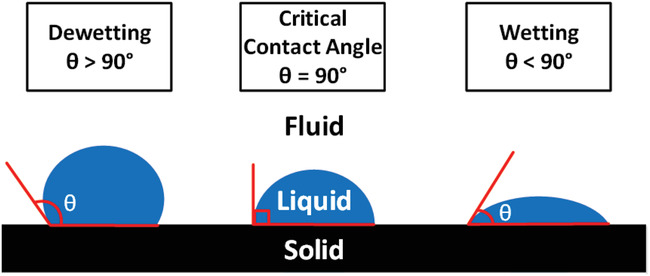
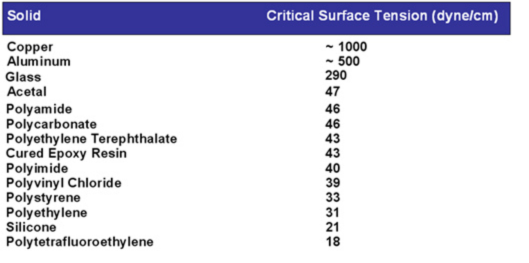
So why does the wax behave differently than the paint in terms of preventing the water to “wet out”? The answer is due to an intrinsic property of every material called surface energy, which is measured by the strength of bonding energies at the surface of any material. A simplified explanation of surface energy is that “unhappy” molecules at the surface do not have a full complement of molecules around them to balance out their attractive forces. These surface molecules therefore exhibit increased adhesive affinity to different molecules that touch the surface. Surface energy can be directly measured for every substance as a function of force per unit length.